
Professor, Former HOD and Former Dean,
Incharge CIL, Department of Pharmaceutical Sciences and Natural Products School of Health Sciences
PhD.
Teaching Experience
15 Years
Research Experience
21 years
| Ph.D. Medicinal Chemistry, NIPER, Mohali | 2007 |
|---|---|
| M.S. (Pharm.) Medicinal Chemistry, NIPER, Mohali | 2002 |
| B. Pharmacy, Pharmaceutical Sciences, M.D.U. Rohtak, Haryana | 2001 |
| Central University of Punjab, Bathinda, Pharmaceutical Sciences– Medicinal Chemistry | 2011-present |
|---|---|
| ISF College of Pharmacy, MOGA, Pharmaceutical Sciences– Medicinal Chemistry | 2009-2011 |
| UMBC, Maryland, USA, Postdoctoral Scientist – Anticancer Research | 2007-2008 |
Administrative Experience
- Head of the Department, PSNP from 4-1-2016 to 3-1-2019 and w.e.f. 1-7-2020 to 30-6-2023
- Dean, School of Health Sciences from 10-11-2020 to 9-11-2023
- In Charge, Central Instrumentation Laboratory (CIL).
- Project In Charge of MOFPI-500 Lakhs
- Member, Standing purchase committees
- Member, IQAC
- Members of Academic Council, Central University of Punjab
Got featured in the list of top 2% international scientists “Updated Science-wide Database of Stanford Citation Indicators” released by Stanford University, USA and published by Elsevier BV on 19th October 2021.
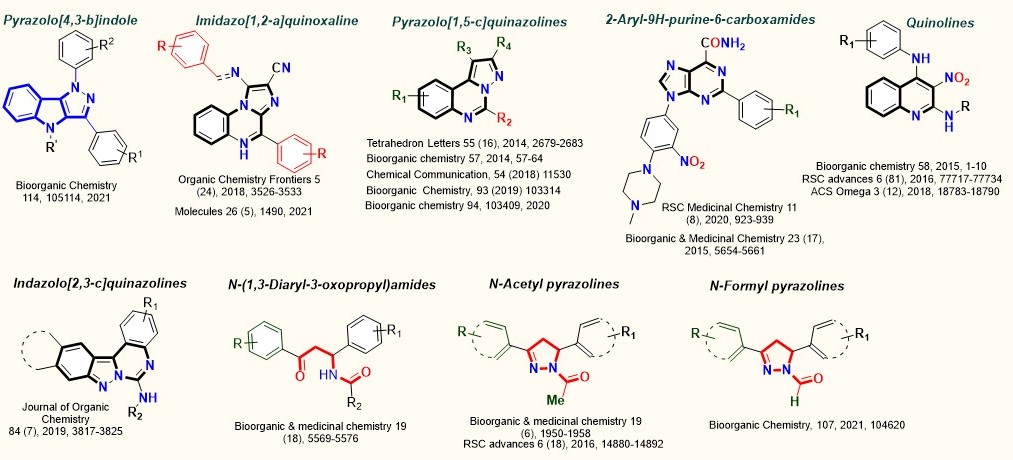
Molecular Degraders
Medicinal Chemistry and Drug Discovery
New chemical entities as EGFR inhibitors, DNA repair inhibitors, Topoisomerase inhibitors and mitochondrial death pathway stimulator, Xanthine Oxidase inhibitors, novel 2-substituted benzoxazole derivatives as COX-2 enzyme inhibitors and PDE-4 inhibitors are developed. RK-33, which first emerged as a DDX-3 inhibitor has a broad spectrum of anticancer effects (Organic Letters 2008, 10, 4681-4684, International Publication Number: WO 2010/039187 (https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010039187), ACS Med. Chem. Lett. 2011, DOI: 10.1021/ml100281b).
https://pubs.acs.org/doi/10.1021/ol8020176
https://pubs.acs.org/doi/10.1021/ml100281b
Diagnostic agents
Pd-catalyzed cascade reaction for the synthesis of fluorescent indazolo[2,3-c]quinazolines with high quantum yield and excellent photostability was developed. Its application is explored in live-cell imaging, which exhibited cytoplasmic and mitochondrial specific staining with no toxicity (J. Org. Chem. 2019, 84, 3817−3825; Patent No. 201811028230).
Green Chemistry and methodology development
Recently a “carbene mediated-Pictet Spengler reaction” for the first time for the synthesis of imidazo[1,2-a]quinoxaline compounds (Org. Chem. Front., 2018, 5, 3526-3533., Indian Patent No. 201611014161) is revealed under Microwave conditions. Novel methodologies were established by using re-usable heterogeneous catalysts such as HClO4 adsorbed on SiO2, HBF4 adsorbed on K-10/KSF clays or Lewis acid catalysts such as Cu(BF4)2·xH2O and Zn(ClO4)2·6H2O with their electrophilic activation potential or water or SDS-water having dual-activation power. Microwave-assisted catalyst-free synthesis of 2-substituted benzoxazoles and 4-aminoquinoline derivatives and pyrazolo(1,5-c)quinazoline were also developed.
- Anticancer Research through exploration of multiple mechanisms including targets such as EGFR, PKM2, Topoisomerases, HDACs, APE1, etc and technology developments including PROTACs and ADCs.
- Xanthine oxidase inhibitors for the treatment of hyperuricemia and gout
- PDE4 inhibitors
- COX-2 inhibitors
A. Completed
· Design, Synthesis and Biological Screening of Novel Multi-target Inhibitors of Tyrosine Kinase(s) and Topoisomerase-I - Department of Science and Technology (DST), Delhi, under the SERB FastTrack Scheme for Young Scientist- (27 Lakhs)
· Design, Synthesis and Biological Screening of Novel Heterocycles as Inhibitors of Dual Tyrosine Kinase(s) and Histone Deacetylase as Potential Anticancer Agents –UGC-Major, Delhi, sanctioned money: Rs/-12,59,000
· Design, synthesis and in vitro screening of mono-/bis- aminoquinolines as EGFR Inhibitors-Research Seed Money, Central University of Punjab, Bathinda: Rs/-3,00,000
· CUPB Fellowship Fund Release (Bristol-Myers Squibb), USA (5,000 Dollars)
B. Ongoing
· New Heterocyclic Ligands as Inhibitors of Multiple EGFR Mutations (L858R/T790M/C797S): Design, Synthesis and Anticancer Evaluation-CSIR extramural research grant Rs. 33 lakhs approximately w.e.f. 2021.
· Project 53803645-CUP PhD. Fellowship funded by Bristol-Myers Squibb), USA (10000 Dollars) w.e.f. 23-8-2019
· Dual Inhibitors of EGFR and HDAC as Anti-Lung Cancer Agents: Design, Synthesis and Biological Evaluation- SERB-Department of Science and Technology (DST), Delhi, under the extramural core research grant- Rs. 4161530, the Year 2018
- Design, Synthesis and Biological Assessment of Novel Anti-Lung Cancer Epidermal Growth Factor Receptor- Proteolysis Targeting Chimeras (EGFR-PROTACs)-SERB, DST 49 Lakhs.-2024
- Consultant to Transasia BioMedical Pvt. Ltd. Mumbai (Workplace AMTZ, Vishakhapatnam): October 2021-present
- Consultant to Bimini Bioetch, Netherlands
- · Life Member-Chemical Research Society of India
- · Life Member-IPGA
- · Life Member-Indian Science Congress
- · Life Member-Indian Society of Chemists and Biologists.
- · Life Member-APTI, India
- · ACS member
- 8th Visitor Award (2023) in Biological Sciences in Cancer Research received from Hon’ble President of India on March 3, 2025.
- · Fellow, Royal Society of Chemistry, London, UK-2022
- Shiv Nath Rai Memorial Mid-Career Best Scientist Award for the year 2022
- ‘Roll of Honour’ by Central University of Punjab in 2021
- · Central University’s Research Award -2019-20 given in the year 2021
- · Central University’s Outstanding Research Award -2018-19 given in the year 2020.
- · Central University’s Research Award -2018-19 given in the year 2020.
- · Central University’s Research Award -2017-18 given in the year 2019.
- · Central University’s Outstanding Research Award -2016-17 given in the year 2018.
- · Central University’s Research Award -2016-17 given in the year 2018.
- · Central University’s Research Award -2015-16 given in the year 2017.
- · Central University’s Research Award -2014-15 given in the year 2016.
- · Most Cited Paper 2004-2007 Award for Tetrahedron Lett.,2005,46, 1721-1724.
- · Most Cited Paper 2005-2008 Award for Tetrahedron Lett.,2005,46, 1721-1724.
- · Qualified National Eligibility Test for Lectureship in Chemical Sciences (CSIR-NET June 2002, conducted by Council for Scientific and Industrial Research, New Delhi, India)
- · Qualified Graduate Aptitude Test for Engineering (GATE 2001) with 99.08 percentile (All India Rank 30); an essential requirement for getting scholarship during M. S. Pharm. course)
- · University Gold Medal in Bachelor of Pharmacy.
- · Member, School Board of the School of Basic and Applied Sciences from 05-01-16-present at Central University of Punjab, Bathinda
- · Expert Member in Board of studies (Biomedical Sciences) from 17-8-2016 – 17-8-2019 at Gurukul Kangri Vishwavidyalaya, Haridwar, India.
- · Chairperson, Board of Studies (Department of Pharmaceutical Sciences and Natural Products) from November 2014- till date at Central University of Punjab, Bathinda.
- · Chairperson on “Socio-legal and Other challenges for the prevention of drug abuse in India: Existing approaches and Agenda of reform” organized by Central University of Punjab, Bathinda, August 24-25, 2017.
- · Expert Member in Board of studies (Pharmacy) and Faculty of Pharmacy from 01-10-2015 – 30-09-2017 at Maharaja Ranjit Singh State Technical University, Bathinda (MRSSTU).
- · Member expert in Institutional Animal Ethics Committee (IAEC) of Maharaja Ranjit Singh Punjab Technical University (CPCSEA Registration. No. 2017/GO/Re/S/18/CPCSEA)
- · Expert Member in Board of studies (Pharmacy) from December 2011- 2013 at PTU, Jalandhar
- · Resource person at 3rd Annual Conference of APTI organized by JCDM College of Pharmacy, Sirsa, Haryana, November 11-12, 2016.
- · Poster Evaluator on 24th ISCB International Conference (ISCB-2018), Frontier Research in Chemistry and Biology, January 11-13, 2018.
- · Poster Evaluator on 69th Indian Pharmaceutical Congress, Chandigarh, December 22-24, 2017.
- · Resource person at 2nd APTI at organized by Govt Polytechnic College, Patiala, Punjab, March18-19, 2016
- · Chairperson at UGC sponsored seminar on “Green Chemistry” organized at GHG Khalsa College, Gursar Sadhar, Punjab, and February 24-25, 2012.
Academia
1. Professor U.C. Banerjee, Department of Pharmaceutical Technology (Biotechnology), NIPER, Mohali
2. Prof. P. V.Bharatam; NIPER, Mohali
3. Prof.Dr. Michael Heuser, Hannover Medical College, Germany
4. Dr. Sandeep Singh, Department of Human Genetics and Molecular Medicine, Central University of Punjab, Bathinda
5. Professor Anil K. Mantha, Department of Animal Sciences, Central University of Punjab, Bathinda
6. Professor Anjana Munshi, Department of Human Genetics and Molecular Medicine, Central University of Punjab, Bathinda
7. Prof. Ramakrishna Wusirika; Department of Biochemistry and Microbiology, Central University of Punjab, Bathinda
8. Dr. Ashoke Sharon; Associate Professor, Department of Chemistry; Birla Institute of Technology, Mesra
9. Dr. Devesh Sawant; Central University of Rajasthan
10. Dr.Umesh Gupta; Central University of Rajasthan
11. Professor Sanjeev Kumar, Department of Botany, Central University of Punjab, Bathinda
12. Dr. Navnath Gawande, Wayne State University College of Pharmacy and Health Sciences, Detroit, USA.
Industrial collaborations
11. Dr. Brahman Pujala; Integral Biosciences, Noida
12. Dr. Hemant Bhutani, Bristol Meyer Squibb, Bangalore
13. Dr. Manoj Chugh, Vice President, Transasia Biomedicals, Pvt. Ltd. Mumbai
15. Dr. Santoshkumar Patil, Senior lead investigator, Syngene Intl. Ltd, India
Papers
- Joshi, G., Yadav, U. P., Rafiq, Z., Grewal, P., Kumar, M., Singh, T., ... & Kumar, R. (2025). Design and Synthesis of Topoisomerases-Histone Deacetylase Dual Targeted Quinoline-Bridged Hydroxamates as Anticancer Agents. Journal of Medicinal Chemistry. https://doi.org/10.1021/acs.jmedchem.4c02135

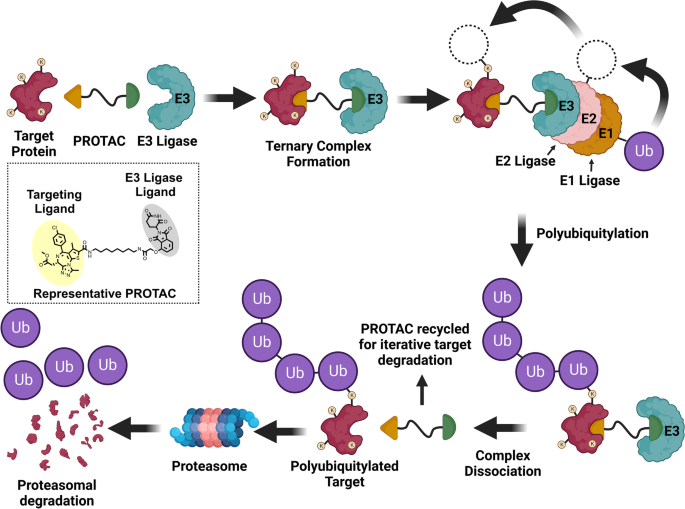
- Kelm, J. K,…… Kumar, R and Gavande, S (2023). PROTAC’ing Oncoproteins: Degraders for Cancer Therapy Targets. Molecular Cancer, 22, 62 (2023) https://doi.org/10.1186/s12943-022-01707-5 (IF: 37).
-
Arora, S., Patra, B., Dhamija, I., Guru, S. K., & Kumar, R. (2025). 2-(4-Bromobenzyl) tethered 4-amino aryl/alkyl-5, 6, 7, 8-tetrahydrobenzo [4, 5] thieno [2, 3-d] pyrimidines: design, synthesis, anticancer assessment via dual topoisomerase-I/II inhibition, and in silico studies. RSC Medicinal Chemistry. https://doi.org/10.1039/D4MD00817K
-
Arora, S., Upadhayay, S., Kumar, P., Kumar, P., & Kumar, R. (2025). Design, synthesis and anticancer evaluation of 4-Substituted 5, 6, 7, 8-tetrahydrobenzo [4, 5] thieno [2, 3-d] pyrimidines as dual topoisomerase I and II inhibitors. Bioorganic Chemistry, 154, 108043. https://doi.org/10.1016/j.bioorg.2024.108043
- Maity, P., Chatterjee, J., Patil, K. T., Arora, S., Katiyar, M. K., Kumar, M.,..& Kumar, R. (2023). Targeting the Epidermal Growth Factor Receptor with Molecular Degraders: State-of-the-Art and Future Opportunities. Journal of Medicinal Chemistry, In Press. (IF: 8.039), DOI: https://doi.org/10.1021/acs.jmedchem.2c01242 66 (5), 3135-3172.

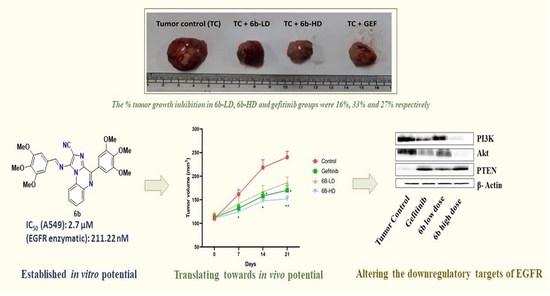
- Kumar, M., Patil, K. T., Maity, P., Chatterjee, J., Singh, T., Joshi, G., ... & Kumar, R. (2024). Design, synthesis, and anticancer assessment of structural analogues of (E)-1-((3, 4, 5-trimethoxybenzylidene) amino)-4-(3, 4, 5-trimethoxyphenyl) imidazo [1, 2-a] quinoxaline-2-carbonitrile (6b), an imidazo [1, 2-a] quinoxaline-based non-covalent EGFR inhibitor. RSC Med. Chem., 2024,15, 2322-2339. https://pubs.rsc.org/en/content/articlelanding/2024/md/d4md00237g
-
Kumar, P., Prasad Yadav, U., Joshi, G., Arora, S., Kumar, M., Chatterjee, J., ... & Kumar, S. (2024). Imidazo [1, 2‐a] Quinoxaline‐2‐Carbonitrile Derivative (RA‐22) Inhibits Self‐Renewal and Growth of Cancer Stem and Cancer Cells via Downregulating AKT Pathway. ChemistrySelect, 9(23), e202400223.

-
Maity, P., Kaitiyar, M. K., Ranolia, A., Joshi, G., Sindhu, J., & Kumar, R. (2024). Unraveling the ability of electronically diverse 9H-carbazoles as potential fluorophores in cellular imaging applications with BSA binding affinities. Journal of Photochemistry and Photobiology A: Chemistry, 115878.

- Bhat, Z. R., Kumar, M., Sharma, N., Yadav, U. P., Singh, T., Joshi, G., ... & Kumar, R. (2022). In Vivo Anticancer Evaluation of 6b, a Non-Covalent Imidazo [1, 2-a] quinoxaline-Based Epidermal Growth Factor Receptor Inhibitor against Human Xenograft Tumor in Nude Mice. Molecules, 27(17), 5540. https://www.mdpi.com/1420-3049/27/17/5540
- Sankar, J., Arora, S., Joshi, G., & Kumar, R. (2022). Pore-forming proteins and their role in cancer and inflammation: Mechanistic insights and plausible druggable targets. Chemico-Biological Interactions, 110127. https://www.sciencedirect.com/science/article/abs/pii/S0009279722003325

- Pahwa, R., Chhabra, J., Kumar, R., & Narang, R. (2022). Melphalan: Recent insights on synthetic, analytical and medicinal aspects. European Journal of Medicinal Chemistry, 114494. https://www.sciencedirect.com/science/article/abs/pii/S0223523422003968

- Katiyar, M. K., Dhakad, G. K., Arora, S., Bhagat, S., Katyal, T., & Kumar, R. (2022). Synthetic Strategies and Pharmacological Activities of Chromene and Its Derivatives: An Overview. Journal of Molecular Structure, 133012. https://www.sciencedirect.com/science/article/abs/pii/S0022286022006731

-
Kaur, M., & Kumar, R. (2022). A Minireview on the Scope of Cadogan Cyclization Reactions Leading to Diverse Azaheterocycles. Asian Journal of Organic Chemistry, e202200092.https://onlinelibrary.wiley.com/doi/abs/10.1002/ajoc.202200092

- Arora, S., Joshi G., Chaturvedi, A., Heuser, M., Patil, S. & Kumar, R. (2021). A Perspective on Medicinal Chemistry Approaches for Targeting Pyruvate Kinase M2. Journal of Medicinal Chemistry, In Press. (IF: 7.45), DOI: 10.1021/acs.jmedchem.1c00981
 https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c00981
https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c00981 - Yadav, U.... & Kumar, R. (2021). Design, Synthesis and Anticancer Activity of 2-Arylimidazo[1,2-a]pyridinyl-3-amines. Bioorganic Chemistry, Just Accepted. (IF: 5.27)
 https://www.sciencedirect.com/science/article/abs/pii/S0045206821008427?via%3Dihub
https://www.sciencedirect.com/science/article/abs/pii/S0045206821008427?via%3Dihub - Kaur, M., Mehta, V., Wani, A. A., Arora, S., Bharatam, P. V., Sharon, A., ... & Kumar, R. (2021). Synthesis of 1, 4-dihydropyrazolo [4, 3-b] indoles via intramolecular C (sp2)-N bond formation involving nitrene insertion, DFT study and their anticancer assessment. Bioorganic Chemistry, 114, 105114. (IF: 5.27)
 https://www.sciencedirect.com/science/article/abs/pii/S0045206821004910?via%3Dihub
https://www.sciencedirect.com/science/article/abs/pii/S0045206821004910?via%3Dihub - Khan, I., Sarkar, B., Joshi, G., Nakhate, K. T., Mantha, A. K., Kumar, R., ... & Gupta, U. (2021). Biodegradable nanoparticulate co-delivery of flavonoid and doxorubicin: Mechanistic exploration and evaluation of anticancer effect in vitro and in vivo. Biomaterials and Biosystems, 3, 100022. https://doi.org/10.1016/j.bbiosy.2021.100022
- Kaur, M., Mehta, V., Arora, S., Munshi, A., Singh, S., & Kumar, R. (2021). Design, Synthesis and Biological Evaluation of New 5‐(2‐Nitrophenyl)‐1‐aryl‐1H‐pyrazoles as Topoisomerase Inhibitors. ChemistrySelect, 6(26), 6644-6651. (IF: 2.109)
 https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.202101459
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.202101459 - Bhutani, P., Joshi, G., Raja, N., Bachhav, N., Rajanna, P. K., Bhutani, H., ... & Kumar, R. (2021). US FDA Approved Drugs from 2015–June 2020: A Perspective. Journal of Medicinal Chemistry, 64, 5, 2339–2381. (IF: 6.205)
 https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01786
https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01786 - Sharma, M., Joshi, G., Arora, S., Singh, T., Biswas, S., Sharma, N., Bhat, Z.R., Tikoo, K., Singh, S., & Kumar, R. (2021). Design and synthesis of Non-Covalent Imidazo[1,2-a]quinoxaline-Based Inhibitors of EGFR and Their Anti-Cancer Assessment. Molecules, 26(5), 1490. (IF: 3.267) https://www.mdpi.com/1420-3049/26/5/1490
- Joshi, G., Sharma, M., Kalra, S., Gavande, N. S., Singh, S., & Kumar, R. (2021). Design, synthesis, biological evaluation of 3, 5-diaryl-4, 5-dihydro-1H-pyrazole carbaldehydes as non-purine xanthine oxidase inhibitors: Tracing the anticancer mechanism via xanthine oxidase inhibition. Bioorganic Chemistry, 107, 104620. (IF: 5.27)
 https://www.sciencedirect.com/science/article/abs/pii/S0045206820319180#ab005
https://www.sciencedirect.com/science/article/abs/pii/S0045206820319180#ab005 - Shalmali, N., Bawa, S., Ali, M. R., Kalra, S., Kumar, R., Zeya, B., ... & Husain, A. (2021) Molecular Docking and In Vitro Anticancer Screening of Synthesized Arylthiazole linked 2H-indol-2-one Derivatives as VEGFR-2 Kinase Inhibitors. Anti-cancer agents in medicinal chemistry. DOI DOI: 10.2174/1871520621666211118102139. (IF: 2.505).
- Kaur, M., & Kumar, R. (2020). Inhibition of Histone Deacetylases, Topoisomerases and Epidermal Growth Factor Receptor by Metal-Based Anticancer Agents: Design & Synthetic Strategies and their Medicinal Attributes. Bioorganic Chemistry, 105, 104396. DOI: https://doi.org/10.1016/j.bioorg.2020.104396. (IF: 5.27)

- Khan, I., Joshi, G., Sarkar, B., Nakhate, K. T., Ajazuddin, Mantha, A. K., ... & Kumar, R. (2020). Doxorubicin and Crocin Co-delivery by Polymeric Nanoparticles for Enhanced Anticancer Potential In Vitro and In Vivo. ACS Appl. Bio Mater., 3, 11, 7789–7799. DOI: https://doi.org/10.1021/acsabm.0c00974

- Kalra, S., Joshi, G., Kumar, M., Arora, S., Kaur, H., Singh, S., ... & Kumar, R. (2020). Anticancer potential of some imidazole and fused imidazole derivatives: exploring the mechanism via epidermal growth factor receptor (EGFR) inhibition. RSC Medicinal Chemistry, 11(8), 923-939. DOI: https://doi.org/10.1039/D0MD00146E.
- Kumar, M., Joshi, G., Chatterjee, J., & Kumar, R. (2020). Epidermal Growth Factor Receptor and its Trafficking Regulation by Acetylation: Implication in Resistance and Exploring the Newer Therapeutic Avenues in Cancer. Current Topics in Medicinal Chemistry, 20(12), 1105-1123. (IF: 3.2189)
- Joshi, G., Kalra, S., Yadav, U. P., Sharma, P., Singh, P. K., Amrutkar, S., ... & Kumar, R. (2020). E-pharmacophore guided discovery of pyrazolo [1, 5-c] quinazolines as dual inhibitors of topoisomerase-I and histone deacetylase. Bioorganic Chemistry, 94, 103409. (IF: 5.27) https://ars.els-cdn.com/content/image/1-s2.0-S0045206819313203-ga1_lrg.jpg

- Kaur, S. Bansal, Y.,Kumar, R., Bansal, G. (2020). A Panoramic Review of IL-6: Structure, Pathophysiological Roles and Inhibitors. Bioorganic & Medicinal Chemistry, 28(5), 115327. (IF: 3.073) https://www.sciencedirect.com/science/article/abs/pii/S096808961931692X?via%3Dihub

- Ansari, A. J., Joshi, G., Yadav, U. P., Maurya, A. K., Agnihotri, V. K., Kalra, S., Kumar, R. & Sawant, D. M. (2019). Exploration of Pd-catalysed four-component tandem reaction for one-pot assembly of pyrazolo [1, 5-c] quinazolines as potential EGFR inhibitors. Bioorganic Chemistry, 103314. (IF: 5.27)https://www.sciencedirect.com/science/article/abs/pii/S0045206819307850?via%3Dihub

- Kumar, R., Saha, N., Purohit, P., Garg, S. K., Seth, K., Meena, V. S., ... & Banerjee, U. C. (2019). Cyclic enaminone as new chemotype for selective cyclooxygenase-2 inhibitory, anti-inflammatory, and analgesic activities. European Journal of Medicinal Chemistry, 182, 111601. (IF: 5.572) https://www.sciencedirect.com/science/article/abs/pii/S0223523419307354?via%3Dihub

- Khan, I..; Joshi, G.; Nakhate, K. T.; Ajazuddin, Kumar, R.; Gupta, U. (2019), Nano-Co-Delivery of Berberine and Anticancer Drug Using PLGA Nanoparticles: Exploration of Better Anticancer Activity and In Vivo Kinetics. Pharmacutical Research,36 (10), 149. (IF: 3.242)
- Ansari, A. J.; Joshi, G.; Sharma, P.; Maurya, A. K.; Metre, R.; Agnihotri, V. K.; Chandaluri, C. G.; Kumar, R*.; Singh, S.; Sawant, D. M. (2019), Pd-Catalyzed four-Component Sequential Reaction Delivers a Modular Fluorophore Platform for Cell Imaging. Journal of Organic Chemistry, DOI: 10.1021/acs.joc.8b02845, 84, 73817-3825. (IF: 4.335)
 https://pubs.acs.org/doi/10.1021/acs.joc.8b02845
https://pubs.acs.org/doi/10.1021/acs.joc.8b02845 - Arora, S.; Joshi, G.; Kalra, S.; Wani, A. A.; Bharatam, P. V.; Kumar, P.; Kumar, R., Knoevenagel/Tandem Knoevenagel and Michael Adducts of Cyclohexane-1,3-dione and Aryl Aldehydes: Synthesis, DFT Studies, Xanthine Oxidase Inhibitory Potential, and Molecular Modeling. ACS Omega 2019, 4 (3), 4604-4614. (IF: 2.87)
 https://pubs.acs.org/doi/10.1021/acsomega.8b03060
https://pubs.acs.org/doi/10.1021/acsomega.8b03060 - Chawla, P.; Kalra, S.; Kumar, R.; Ranjit, S.; Saraf, S. (2019), Novel 2-(substituted phenyl Imino)-5-benzylidene-4-thiazolidinones as possible non-ulcerogenic tri-action drug candidates: synthesis, characterization, biological evaluation And docking studies. Medicinal Chemistry Research. 28 (3), 340–359. (IF: 1.783)
- Chhokar, N., Kalra, S., Chauhan, M., Munshi, A., &Kumar, R.* (2019). Quinoline-based protein-protein interaction inhibitors of LEDGF/p75 and HIV integrase: An in silico study. Current Topics in Medicinal Chemistry. Doi: 10.2174/1568026619666190208164801. (IF: 3.218) https://www.eurekaselect.com/article/96528

- Narang, R., Kumar, R., Kalra, S., Nayak, S. K., Khatik, G. L., Kumar, G. N., ... & Singh, S. K. (2019). Recent advancements in mechanistic studies and structure activity relationship of FoF1 ATP synthase inhibitor as antimicrobial agent. European Journal of Medicinal Chemistry, 182, 111644. (IF: 5.572)
- Kalra, S., Joshi, G., Kumar, R., & Munshi, A. (2018). Role of 2-Dimensional Autocorrelation Descriptors in Predicting Antimalarial Activity of Artemisinin and its Aanalogues: A QSAR Study. Current Topics in Medicinal Chemistry, 18(31), 2720-2730. (IF: 3.218)
- Joshi, G., Wani, A, Sharma S., Bhuatni, P., Bharatam, P.,Paul, A. T.,Kumar, R.* (2018). Unanticipated Cleavage of 2‑Nitrophenyl-Substituted N‑Formyl Pyrazolines under Bechamp Conditions: Unveiling the Synthesis of 2‑Aryl Quinolines and Their Mechanistic Exploration via DFT Studies. ACS Omega, 3 (12), 18783-18790. (IF: 2.87) https://pubs.acs.org/doi/10.1021/acsomega.8b02682

- Joshi, G., Chauhan, M, Kumar, R., Thakur, A., Sharma, S., Singh, R., Wani, A, Sharon, A., Bharatam, P.,Kumar, R.* (2018). Cyclocondensation reactions of an electron deactivated 2- aminophenyl tethered imidazole with mono/1,2-biselectrophiles: Synthesis and DFT studies on rationalisation of imidazo[1,2-a]quinoxaline versus benzo[f]imidazo[1,5-a][1,3,5]triazepine selectivity switch. Organic Chemistry Frontiers,5, 3526-3533. (IF: 5.281) https://pubs.rsc.org/en/content/articlelanding/2018/qo/c8qo00706c
- Sawant,D. M., Sharma, S., Pathare, R. S., Joshi, G, Kalra, S., Sukanya, Maurya, A. K., Metre, R. K., Agnihotri, V. K., Khan, S., Kumar, R.*Pardasani, RT. (2018). Relay Tricyclic Pd (II)/Ag (I) Catalysis: Design of a Four-Component Reaction Driven by Nitrene-Transfer on Isocyanide Yields Inhibitors of EGFR. Chemical Communication, 2018, 54, 11530--11533. (IF: 5.996)
https://doi.org/10.1039/C8CC05845H
- Chokkar, N., Kalra, S., Chauhan, M., Kumar, R.* (2018). A review on quinoline derived scaffolds as Anti-HIV Agents. Mini-Reviews in Medicinal Chemistry, DOI:10.2174/1389557518666181018163448. (IF: 2.733)
- Kaur, G., Cholia, R. P., Joshi, G., Amrutkar, S. M., Kalra, S., Mantha, A. K., ... &Kumar, R. (2018). Anticancer activity of dihydropyrazolo [1, 5-c] quinazolines against rat C6 glioma cells via inhibition of topoisomerase II. Archiv der Pharmazie, 351(6), 1800023. (IF: 2.59) https://onlinelibrary.wiley.com/doi/epdf/10.1002/ardp.201800023
- Cholia, R. P., Dhiman, M., Kumar, R.,& Mantha, A. K. (2018). Oxidative stress stimulates invasive potential in rat C6 and human U-87 MG glioblastoma cells via activation and cross-talk between PKM2, ENPP2 and APE1 enzymes. Metabolic brain disease, 1-20. (IF: 2.726)
- Kalra, S., Kaur, R.P., Ludhiadch, A., Shafi, G., Vashista, R., Kumar, R. and Munshi, A., 2018. Association of CYP2C19* 2 and ALDH1A1* 1/* 2 variants with disease outcome in breast cancer patients: results of a global screening array. European journal of clinical pharmacology, 74(10), pp.1291-1298. (IF: 2.641)
- Sharma, S., Joshi, G., Kalra, S., Singh, S. and Kumar, R.,(2018). Synthetic versus Enzymatic Pictet-Spengler Reaction: An Overview. Current Organic Synthesis, 15(7), pp.924-939. (IF: 1.983) https://www.eurekaselect.net/article/91095

- Kaur, M., and Kumar, R. (2018). C‐N and N‐N bond formation via Reductive Cyclization: Progress in Cadogan /Cadogan‐Sundberg Reaction.ChemistrySelect, 3, 5330 – 5340. (IF: 2.012)
 https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.201800779
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.201800779 - Kumar, R., Joshi, G., Kler, H., Kalra, S., Kaur, M. and Arya, R., 2018. Toward an understanding of structural insights of xanthine and aldehyde oxidases: An overview of their inhibitors and role in various diseases. Medicinal research reviews, 38(4), pp.1073-1125. (IF: 9.3)
- Kalra, S., Joshi, G., Munshi, A., Kumar, R., (2017). Structural insights of cyclin dependent kinases: Implications in design of selective inhibitors. European Journal of Medicinal Chemistry 142, 424-458. (IF: 5.572) https://www.sciencedirect.com/science/article/abs/pii/S0223523417306827?via%3Dihub#abs0015

- G. Joshi, H. Nayyar, S. Kalra, P. Sharma, A. Munshi, S. Singh, R. Kumar,(2017). Pyrimidine Containing Epidermal Growth Factor Receptor Kinase inhibitors: Synthesis and Biological Evaluation, Chemical Biology & Drug Design, doi:10.1111/cbdd.13027. (IF: 2.548)
- Cholia, R.P., Kumari, S., Kumar, S., Kaur, M., Kaur, M., Kumar, R., Dhiman, M. and Mantha, A.K., 2017. An in vitro study ascertaining the role of H2O2 and glucose oxidase in modulation of antioxidant potential and cancer cell survival mechanisms in glioblastoma U-87 MG cells. Metabolic brain disease, 32(5), pp.1705-1716. (IF: 2.726)
- Muraleedharan, A., Joshi, G. and Kumar, R.,2017. Natural Products Based Ayurvedic Formulations: Chemical Cons tituents and Treatment in Neurodegenerative Disorders 130. Mini-Reviews in Organic Chemistry, 14(4), pp.280-287. (IF: 1.824)
- M. Garg, M. Chauhan, R. Kumar, Identification of new insulin growth factor receptor-1 (IGF-1R) inhibitors via exploring ATPas kinase domain of IGF-1R through virtual screening, Medicinal Chemistry Research, 26 (2017) 205-219. (IF: 1.783)
- P.K. Singh, A. Negi, P.K. Gupta, M. Chauhan, R. Kumar, Toxicophore exploration as a screening technology for drug design and discovery: techniques, scope and limitations, Archives of toxicology, 90 (2016) 1785-1802. (IF: 5.059)
- G. Joshi, H. Nayyar, J. Marin Alex, G. S Vishwakarma, S. Mittal, R. Kumar, Pyrimidine-fused Derivatives: Synthetic Strategies and Medicinal Attributes, Current topics in medicinal chemistry,16 (2016) 3175-3210. (IF: 3.218)
- G. Joshi, S.M. Amrutkar, A.T. Baviskar, H. Kler, S. Singh, U.C. Banerjee, R. Kumar, Synthesis and biological evaluation of new 2, 5-dimethylthiophene/furan based N-acetyl pyrazolines as selective topoisomerase II inhibitors, RSC Advances, 6 (2016) 14880-14892. (IF: 3.119)
- B.S. Gill, P. Sharma, R. Kumar, S. Kumar, Misconstrued versatility of Ganoderma lucidum: a key player in multi-targeted cellular signaling, Tumor Biology, 37 (2016) 2789-2804. (IF: 3.650)
- M. Chauhan, G. Sharma, G. Joshi, R. Kumar, Epidermal Growth Factor Receptor (EGFR) and its Cross-Talks with Topoisomerases: Challenges and Opportunities for Multi-Target Anticancer Drugs, Current pharmaceutical design, 22 (2016) 3226-3236. (IF: 2.208)
- M. Chauhan, G. Joshi, H. Kler, A. Kashyap, S.M. Amrutkar, P. Sharma, K.D. Bhilare, U.C. Banerjee, S. Singh, R. Kumar, Dual inhibitors of epidermal growth factor receptor and topoisomerase IIα derived from a quinoline scaffold, RSC Advances, 6 (2016) 77717-77734. (IF: 3.119)
- Rana, J.M. Alex, M. Chauhan, G. Joshi, R. Kumar, A review on pharmacophoric designs of antiproliferative agents, Medicinal Chemistry Research, 24 (2015) 903-920. (IF: 1.783)
- R. P Cholia, H. Nayyar, R. Kumar, A. K Mantha, Understanding the multifaceted role of ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) and its altered behaviour in human diseases, Current molecular medicine, 15 (2015) 932-943. (IF: 1.600)
- Negi, J.M. Alex, S.M. Amrutkar, A.T. Baviskar, G. Joshi, S. Singh, U.C. Banerjee, R. Kumar, Imine/amide–imidazole conjugates derived from 5-amino-4-cyano-N1-substituted benzyl imidazole: Microwave-assisted synthesis and anticancer activity via selective topoisomerase-II-α inhibition, Bioorganic & Medicinal Chemistry, 23 (2015) 5654-5661. (IF: 3.073)

- R. Kumar, C. Santos Dos, T.S. Ahluwalia, S. Singh, Editorial Signal Transduction Inhibitors as Promising Anticancer Agents, BioMed Research International, (2015). doi: 10.1155/2015/584170. (IF: 2.140)
- A.N. Gaurav Joshi, Pankaj Kumar Singh, Sandeep Singh, Raj Kumar, Growth factors mediated cell signalling in prostate cancer progression: Implications in discovery of anti-prostate cancer agents, Chemico-biological interactions, 240 (2015) 120-133. (IF: 3.723)
 https://www.sciencedirect.com/science/article/abs/pii/S0009279715300399?via%3Dihub
https://www.sciencedirect.com/science/article/abs/pii/S0009279715300399?via%3Dihub - M. Garg, M. Chauhan, P.K. Singh, J.M. Alex, R. Kumar, Pyrazoloquinazolines: Synthetic strategies and bioactivities, European journal of medicinal chemistry, 97 (2015) 444-461. (IF: 5.572)
- M. Chauhan, A. Rana, J.M. Alex, A. Negi, S. Singh, R. Kumar, Design, microwave-mediated synthesis and biological evaluation of novel 4-aryl (alkyl) amino-3-nitroquinoline and 2, 4-diaryl (dialkyl) amino-3-nitroquinolines as anticancer agents, Bioorganic chemistry, 58 (2015) 1-10. (IF: 5.27) https://www.sciencedirect.com/science/article/abs/pii/S0045206814001102

- M. Chauhan, R. Kumar, A comprehensive review on bioactive fused heterocycles as purine-utilizing enzymes inhibitors, Medicinal Chemistry Research, 24 (2015) 2259-2282. (IF: 1.783)
- K. Seth, S.K. Garg, R. Kumar, P. Purohit, V.S. Meena, R. Goyal, U.C. Banerjee, A.K. Chakraborti, 2-(2-Arylphenyl) benzoxazole as a novel anti-inflammatory scaffold: synthesis and biological evaluation, ACS medicinal chemistry letters, 5 (2014) 512-516. (IF: 3.975) https://pubs.acs.org/doi/10.1021/ml400500e

- D. Kumar, R. Kumar, Microwave-assisted synthesis of pyrazolo [1, 5-c] quinazolines and their derivatives, Tetrahedron Letters, 55 (2014) 2679-2683. (IF: 2.275) https://www.sciencedirect.com/science/article/abs/pii/S0040403914004353?via%3Dihub

- D. Kumar, G. Kaur, A. Negi, S. Kumar, S. Singh, R. Kumar, Synthesis and xanthine oxidase inhibitory activity of 5, 6-dihydropyrazolo/pyrazolo [1, 5-c] quinazoline derivatives, Bioorganic Chemistry, 57 (2014) 57-64. (IF: 5.27) https://www.sciencedirect.com/science/article/abs/pii/S0045206814000741

- G. Kaur, R.P. Cholia, A.K. Mantha, R. Kumar, DNA repair and redox activities and inhibitors of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1): a comparative analysis and their scope and limitations toward anticancer drug development: Miniperspective, Journal of Medicinal Chemistry, 57 (2014) 10241-10256. (IF: 6.205)
 https://pubs.acs.org/doi/10.1021/jm500865u
https://pubs.acs.org/doi/10.1021/jm500865u - J.M. Alex, S. Singh, R. Kumar, 1‐Acetyl‐3, 5‐diaryl‐4, 5‐dihydro (1H) pyrazoles: exhibiting anticancer activity through intracellular ROS scavenging and the mitochondria‐dependent death pathway, Archiv der Pharmazie, 347 (2014) 717-727. (IF: 2.59)
- J.M. Alex, R. Kumar, 4, 5-Dihydro-1 H-pyrazole: an indispensable scaffold, Journal of enzyme inhibition and medicinal chemistry, 29 (2014) 427-442. (IF: 4.673)
- Negi, P. Ramarao, R. Kumar, Recent advancements in small molecule inhibitors of insulin–like growth factor-1 receptor (IGF-1R) tyrosine kinase as anticancer agents, Mini reviews in medicinal chemistry, 13 (2013) 653-681. (IF: 2.733)
- Negi, S. Bhushan, P. Gupta, P. Garg, R. Kumar, Cystathionine β-Lyase-Like Protein with Pyridoxal Binding Domain Characterized in Leishmania major by Comparative Sequence Analysis and Homology Modelling, ISRN Computational Biology, 2013 (2013) doi: 10.1155/2013/520435.
- D. Kumar, D.N. Kommi, R. Chebolu, S.K. Garg, R. Kumar, A.K. Chakraborti, Selectivity control during the solid supported protic acids catalysed synthesis of 1, 2-disubstituted benzimidazoles and mechanistic insight to rationalize selectivity, RSC Advances, 3 (2013) 91-98. (IF: 3.119)
- M. Chauhan, R. Kumar, Medicinal attributes of pyrazolo [3, 4-d] pyrimidines: a review, Bioorganic & Medicinal Chemistry, 21 (2013) 5657-5668. (IF: 3.073) https://www.sciencedirect.com/science/article/abs/pii/S0968089613006470
- A.T. Baviskar, U.C. Banerjee, M. Gupta, R. Singh, S. Kumar, M.K. Gupta, S. Kumar, S.K. Raut, M. Khullar, S. Singh, Synthesis of imine-pyrazolopyrimidinones and their mechanistic interventions on anticancer activity, Bioorganic & Medicinal Chemistry, 21 (2013) 5782-5793. (IF: 3.073)
 https://www.sciencedirect.com/science/article/abs/pii/S0968089613006275
https://www.sciencedirect.com/science/article/abs/pii/S0968089613006275 - S.S. Malhi, A. Budhiraja, S. Arora, K.R. Chaudhari, K. Nepali, R. Kumar, H. Sohi, R.S. Murthy, Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes, International journal of pharmaceutics, 432 (2012) 63-74. (IF: 4.845)
- S. Kumar, S. Sapra, R. Kumar, M.K. Gupta, S. Koul, T. Kour, A.K. Saxena, O.P. Suri, K.L. Dhar, Synthesis of combretastatin analogs: evaluation of in vitro anticancer activity and molecular docking studies, Medicinal Chemistry Research, 21 (2012) 3720-3729. (IF: 1.783)
- N. Kumar, K. Nepali, S. Sapra, K.R.V. Bijjem, R. Kumar, O.P. Suri, K.L. Dhar, Effect of nitrogen insertion on the antitussive properties of menthol and camphor, Medicinal Chemistry Research, 21 (2012) 531-537. (IF: 1.783)
- K. Nepali, G. Singh, A. Turan, A. Agarwal, S. Sapra, R. Kumar, U.C. Banerjee, P.K. Verma, N.K. Satti, M.K. Gupta, A rational approach for the design and synthesis of 1-acetyl-3, 5-diaryl-4, 5-dihydro (1H) pyrazoles as a new class of potential non-purine xanthine oxidase inhibitors, Bioorganic & Medicinal Chemistry, 19 (2011) 1950-1958. (IF: 3.073)
 https://pubmed.ncbi.nlm.nih.gov/21353569/
https://pubmed.ncbi.nlm.nih.gov/21353569/ - K. Nepali, A. Agarwal, S. Sapra, V. Mittal, R. Kumar, U.C. Banerjee, M.K. Gupta, N.K. Satti, O.P. Suri, K.L. Dhar, N-(1, 3-Diaryl-3-oxopropyl) amides as a new template for xanthine oxidase inhibitors, Bioorganic & Medicinal Chemistry, 19 (2011) 5569-5576. (IF: 3.073)
 https://www.sciencedirect.com/science/article/abs/pii/S0968089611005888?via%3Dihub
https://www.sciencedirect.com/science/article/abs/pii/S0968089611005888?via%3Dihub - R. Kumar, Darpan, S. Sharma, R. Singh, Xanthine oxidase inhibitors: a patent survey, Expert opinion on therapeutic patents, 21 (2011) 1071-1108. (IF: 5.611) https://www.tandfonline.com/doi/figure/10.1517/13543776.2011.577417?scroll=top&needAccess=true
- S. Sapra, K. Nepali, R. Kumar, R. Goyal, O.P. Suri, V.K. Koul, K.L. Dhar, Analysis of Mentha waste products using GC-MS, Int. J. Pharm. Sci. Res, 1 (2010) 53-55.
- H.S. Sandhu, S. Sapra, M. Gupta, K. Nepali, R. Gautam, S. Yadav, R. Kumar, S.M. Jachak, M. Chugh, M.K. Gupta, Synthesis and biological evaluation of arylidene analogues of Meldrum’s acid as a new class of antimalarial and antioxidant agents, Bioorganic & Medicinal Chemistry, 18 (2010) 5626-5633. (IF: 3.073)
 https://www.sciencedirect.com/science/article/abs/pii/S0968089610005614
https://www.sciencedirect.com/science/article/abs/pii/S0968089610005614 - Kondaskar, S. Kondaskar, R. Kumar, J.C. Fishbein, N. Muvarak, R.G. Lapidus, M. Sadowska, M.J. Edelman, G.M. Bol, F. Vesuna, Novel, broad spectrum anticancer agents containing the tricyclic 5: 7: 5-fused diimidazodiazepine ring system, ACS medicinal chemistry letters, 2 (2010) 252-256. (IF: 3.975)
- A.K. Chakraborti, S.K. Garg, R. Kumar, H.F. Motiwala, P.S. Jadhavar, Progress in COX-2 inhibitors: a journey so far, Current medicinal chemistry, 17 (2010) 1563-1593. (IF: 4.184)
- G. Sharma, R. Kumar, A.K. Chakraborti, Fluoroboric acid adsorbed on silica-gel (HBF 4–SiO 2) as a new, highly efficient and reusable heterogeneous catalyst for thia-Michael addition to α, β-unsaturated carbonyl compounds, Tetrahedron Letters,49 (2008) 4272-4275. (IF: 2.275)
- G. Sharma, R. Kumar, A.K. Chakraborti, ‘On water’synthesis of 2, 4-diaryl-2, 3-dihydro-1, 5-benzothiazepines catalysed by sodium dodecyl sulfate (SDS), Tetrahedron Letters, 49 (2008) 4269-4271. (IF: 2.275)
- R. Kumar, R.K. Ujjinamatada, R.S. Hosmane, The first synthesis of a novel 5: 7: 5-fused diimidazodiazepine ring system and some of its chemical properties, Organic Letters, 10 (2008) 4681-4684. (IF: 6.091)
- D. Kumar, R. Kumar, A.K. Chakraborti, Tetrafluoroboric acid adsorbed on silica gel as a reusable heterogeneous dual-purpose catalyst for conversion of aldehydes/ketones into acetals/ketals and back again, Synthesis, 2008 (2008) 1249-1256. (IF: 2.867)
- G. Sharma, R. Kumar, A.K. Chakraborti, A novel environmentally friendly process for carbon–sulfur bond formation catalyzed by montmorillonite clays, Journal of Molecular Catalysis A: Chemical, 263 (2007) 143-148. (IF: 3.687)
- H.F. Motiwala, R. Kumar, A.K. Chakraborti, Microwave-accelerated solvent-and catalyst-free synthesis of 4-aminoaryl/alkyl-7-chloroquinolines and 2-aminoaryl/alkylbenzothiazoles, Australian journal of chemistry, 60 (2007) 369-374. (IF: 1.226)
- R. Kumar, D. Kumar, A.K. Chakraborti, Perchloric acid adsorbed on silica gel (HClO4-SiO2) as an inexpensive, extremely efficient, and reusable dual catalyst system for acetal/ketal formation and their deprotection to aldehydes/ketones, Synthesis, 2007 (2007) 299-303. (IF: 2.867)
- G.L. Khatik, G. Sharma, R. Kumar, A.K. Chakraborti, Scope and limitations of HClO 4–SiO 2 as an extremely efficient, inexpensive, and reusable catalyst for chemoselective carbon–sulfur bond formation, Tetrahedron, 63 (2007) 1200-1210. (IF: 2.233)
- G.L. Khatik, R. Kumar, A.K. Chakraborti, Magnesium perchlorate as a new and highly efficient catalyst for the synthesis of 2, 3-dihydro-1, 5-benzothiazepines, Synthesis, 2007 (2007) 541-546. (IF: 2.867)
- H. Bhutani, S. Singh, S. Vir, K. Bhutani, R. Kumar, A.K. Chakraborti, K. Jindal, LC and LC-MS study of stress decomposition behaviour of isoniazid and establishment of validated stability-indicating assay method, Journal of Pharmaceutical and Biomedical analysis, 43 (2007) 1213-1220. (IF: 3.209)
- R. Kumar, R. Thilagavathi, R. Gulhane, A.K. Chakraborti, Zinc (II) perchlorate as a new and highly efficient catalyst for formation of aldehyde 1, 1-diacetate at room temperature and under solvent-free conditions, Journal of Molecular Catalysis A: Chemical, 250 (2006) 226-231. (IF: 3.687)
- R.K. Khunger, Cu (BF4) 2· xH2O: A Versatile Catalyst, Synlett, 2006 (2006) 327-328. (IF: 2.006)
- G.L. Khatik, R. Kumar, A.K. Chakraborti, Catalyst-free conjugated addition of thiols to α, β-unsaturated carbonyl compounds in water, Organic Letters, 8 (2006) 2433-2436. (IF: 6.091)
- R. Thilagavathi, R. Kumar, V. Aparna, M.E. Sobhia, B. Gopalakrishnan, A.K. Chakraborti, Three-dimensional quantitative structure (3-D QSAR) activity relationship studies on imidazolyl and N-pyrrolyl heptenoates as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) inhibitors by comparative molecular similarity indices analysis (CoMSIA), Bioorganic & Medicinal Chemistry Letters, 15 (2005) 1027-1032. (IF: 2.572)
- R. Kumar, C. Selvam, G. Kaur, A.K. Chakraborti, Microwave-assisted direct synthesis of 2-substituted benzoxazoles from carboxylic acids under catalyst and solvent-free conditions, Synlett, (2005) 1401-1404. (IF: 2.006)
- R. Kumar, A.K. Chakraborti, Copper (II) tetrafluoroborate as a novel and highly efficient catalyst for acetal formation, Tetrahedron letters, 46 (2005) 8319-8323. (IF: 2.275)
- S.K. Garg, R. Kumar, A.K. Chakraborti, Zinc perchlorate hexahydrate catalysed conjugate addition of thiols to α, β-unsaturated ketones, Synlett, 2005 (2005) 1370-1374. (IF: 2.006)
- S.K. Garg, R. Kumar, A.K. Chakraborti, Copper (II) tetrafluoroborate as a novel and highly efficient catalyst for Michael addition of mercaptans to α, β-unsaturated carbonyl compounds, Tetrahedron letters, 46 (2005) 1721-1724. (IF: 2.275)
- A.K. Chakraborti, R. Thilagavathi, R. Kumar, Copper (II) tetrafluoroborate-catalyzed formation of aldehyde-1, 1-diacetates, Synthesis, 2004 (2004) 831-833. (IF: 2.867)
Books
1. Edited; Topoisomerase Inhibitors: Classification, Mechanisms of Action and Adverse Effects, 2017 Editors: Raj Kumar and Sandeep Singh; Nova Publisher, USA Inc., ISBN: 978-1-53611-841-4.  https://novapublishers.com/shop/topoisomerase-inhibitors-classification-mechanisms-of-action-and-adverse-effects/
https://novapublishers.com/shop/topoisomerase-inhibitors-classification-mechanisms-of-action-and-adverse-effects/
2. Pharmaceutical Organic Chemistry for B. Pharmacy students by S.C. Sharma and Raj Kumar, Vishal Publishing Co., Jalandhar, India, ISBN 978-81-921432-9-3. 
Book Chapters
1. Joshi, G., Kaur, M., & Kumar, R. Dynamic Axial Chirality in Drug Design and Discovery: Introduction to Atropisomerism, Classification, Significance, Recent Trends and Challenges. Drug Discovery and Development: From Targets and Molecules to Medicines, 2021, 103, Springer Nature. 
2. Arora, S., Kumar, M., Joshi, G. & Kumar, R. Chapter 6, The Future of COVID-19, Treatment COVID-19: Diagnosis and Management-II, 2021, 168-199. Bentham Science Publishers Ltd. https://www.eurekaselect.com/chapter/15050
3. Joshi, G., Thakur, A., Kaur, G., Kalra, S., Singh, S. and Kumar, R., 2017. Interaction between topoisomerases and histone deacetylases: Role in cancer progression and therapeutic interventions. Nova Science Publishers, Inc.
4. Kaur, G., Joshi, G., Sharma, P., Kumar R., and Singh S., 2017. Topoisomerases Genetics and Its associated diseases. Nova Science Publishers, Inc
Thesis Supervision
(a) M. Pharm. thesis Supervised: 35
(b) M. Sc. Dissertation/Project work Supervised: 13
(c) PhD students supervised-4, under Supervision-3, under co- supervision-1
M. Pharm. thesis
|
1. |
Title: “Design and Synthesis of 1-Acetyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazoles as a New Class of Non-purine Xanthine Oxidase Inhibitors”- M. Pharm. degree awarded to Mr. Anil Turan (MDU Regn No: 99-RUR-246) in August 2010 |
|
2. |
Title: “Design and Synthesis of Some New Purine based Allopurinol Analogues as Xanthine Oxidase Inhibitors”- M. Pharm. degree awarded to Mr. Mukesh Gupta (PTU Regn No: 80404446008) in August 2010 |
|
3. |
Title: “Synthesis and Biological Evaluation of Arylidene Analogues of Meldrum’s acid as a New Class of Antimalarial and Antioxidant Agents”- M. Pharm. degree awarded to Mr. Harmeet Singh Sandhu (PTU Regn No: 80404446002) in August 2010 |
|
4. |
Title: “Synthesis and Biological Evaluation of Novel 5:6/5:7:6-Fused Heterocycles as AnticancerAgents”- M. Pharm. degree awarded to Mr. Rajveer Singh (PTU Regn No: 96065181126) in August 2011 |
|
5. |
Title: “Synthesis and Evaluation of New N-Heterocycles as Potential Anticancer Agents and Xanthine Oxidase Inhibitors”- M. Pharm. degree awarded to Mr. Darpan (PTU Regn No: 96065181116) in August 2011 |
|
6. |
Title: “Synthesis and Biological Evaluation of Novel Heterocycles as Potential Anticancer Agents”- M. Pharm. degree awarded to Mr. Sahil Sharma (PTU Regn No: 96065181128) in August 2011 |
|
7. |
Title: “Imidazole based compounds: Synthesis and in vitro anticancer screening”- M. Pharm. , Thesis submitted in August 2013 by Mr. Arvind Negi (Central University of Punjab Regn No: CUP/Mphm-PhD/SBAS/CPS/2011/01) |
|
8. |
Title: “Synthesis and Evaluation of Novel Heterocyclics as Anticancer Agents”- M. Pharm. , Thesis submitted in August 2013 by Miss Monika Chauhan (Central University of Punjab Regn No: CUP/Mphm-PhD/SBAS/CPS/2011/04) |
|
9. |
Title: “Synthesis and Biochemical Screening of Novel Non-Purine Based Xanthine Oxidase Inhibitors”- M. Pharm. Thesis submitted in August 2013 by Mr. Deependra Kumar (Central University of Punjab Regn No: CUP/Mphm-PhD/SBAS/CPS/2011/06) |
|
10. |
Title: “Anticancer potential of new N-acetyl pyrazoline derivatives of 1, 3-diaryl/heteroarylpropenones: Synthesis and evaluation”- M. Pharm., Thesis submitted in August 2013 by MissJimi Marin Alex (Central University of Punjab Regn No: CUP/M.Pham-Ph.D/SBAS/CPS/2011-12/02) |
|
11. |
Title: “Synthesis of mono- and bis-aminoquinoline compounds as potential anticancer agents”-Mr. Anil Rana (Central University of Punjab Regn No: CUPB/M.PHARM-MC/SBAS/CPS/2012-13/08). Thesis submitted in August 2014 |
|
12. |
Title: “Design and synthesis of pyrazolo[1,5-c] quinazoline based anticancer agents”- Mr. GauravJoshi (Central University of Punjab Regn No: CUPB/M-PHARM-MC/SBAS/CPS/2012-13/02). A thesis submitted in August 2014 |
|
13. |
Title: “Design and synthesis of ape1 inhibitors as putative anticancer agents”- Miss GagandeepKaur (Central University of Punjab Regn No: CUPB/M-PHARM-MC/SBAS/CPS/2012-13/04). Thesis submitted in August 2014 |
|
14. |
Title: “Synthesis and biological evaluation of pyrazoloquinazoline scaffolds as putative anti-cancer agents”- Candidate: Mr. Pankaj Kumar Singh (CUPB/MPharm-MC/SBAS/CPS/2013-14/06). Thesis submitted in August 2015 |
|
15. |
Title: “Synthesis and biological evaluation of pyrimidine based analogues as anticancer agents”-Candidate: Mr. Himanshu Nayyar (CUPB/MPharm-MC/SBAS/CPS/2013-14/08).Thesis submitted in August 2015 |
|
16. |
Title: “Synthesis and evaluation of quinolone based compounds as putative anticancer agents”-Candidate: Ms. Archna Kashyap (CUPB/MPharm-MC/SBAS/CPS/2013-14/01). Thesis submitted in August 2015 |
|
17. |
Title: “Synthesis of condensed Pyrazolo[1,5-c]quinazolines and their evaluation as putative anticancer agents”-Candidate: Mr. Gourav Sharma (CUPB/MPharm-MC/SBAS/CPS/2014-15/03). Thesis submitted in August 2016 |
|
18. |
Title: “Design and Synthesis of imidazo[1,2-a]quinoxaline derivatives as putative anti-proliferative agents”-Candidate: Rakesh Kumar (Reg. No. 15mpharm05). Thesis submitted in 2017 |
|
19. |
Title: “Synthesis and In Vitro Screening of N-FormylatedPyrazolines as Xanthine Oxidase Inhibitors”-Candidate: Manisha Sharma (Reg. No. 15mpharm07). Thesis submitted in 2017 |
|
20. |
Title: “Imidazole fused heterocycles as putative anticancer agents”-Candidate: Ankush Thakur (Reg. No. 15mpharm03). Thesis submitted in 2017 |
|
21. |
Title: “Imidazole containing new heterocycles as putative anticancer agents”-Candidate: Sachin Sharma (Reg. No. 16mpharm01). Thesis submitted in 2018 |
|
22. |
Title: “Antiproliferative Activity of Chloroform andMethanol Extracts of Piper attenuatum(Buch-Ham)”-Candidate: Neha Pathak (Reg. No. 16mphyto04). Thesis submitted in 2018 |
|
23. |
Title: “Synthesis and antiproliferative activity of pyrazole based heterocycles”-Candidate: Vishakha Pandey (Reg. No. 16mpharm06). Thesis submitted in 2018 |
|
24. |
Title: “Synthesis of New Nitrogen-Containing Heterocycles as Anti-proliferative agents”-Candidate: Sajal Biswas (Reg. No. 17mpharm10). Thesis submitted in 2019. |
|
25. |
Title: “Synthesis of Benzamides as Antileishmanial Agents”-Candidate: Surya Shuvam (Reg. No. 17mphyto06). Thesis submitted in 2019 |
|
26. |
Title: “Imidazo[1,2-a]quinoxalines as new anti-proliferative agents”-Candidate: Joydeep Chatterjee (Reg. No. 18mpharm04). Thesis submitted in 2020 |
|
27. |
Title: “Virtual screening and synthesis of imidazole fused quinoxaline based EGFR inhibitors as putative anti-proliferative agents”-Candidate: Loveleen (Reg. No. 18mpharm16). Thesis submitted in 2020 |
|
28. |
Title: “Identification of inhibitors of pyruvate kinase M2(PKM2) as potential anticancer agents: An in silico approach” Merugumala Kusuma (Reg. No. 18mpharm01). Thesis submitted in 2020 |
|
29. |
Title: “Isolation and characterization of piperine from Piper nigrum and exploration of in silico binding affinities of piperine and its derivatives towards pteridine reductase-1 (PTR-1) as a leishmanial drug target” Kumar P. (Reg. No. 18mphyto09). Thesis submitted in 2020 |
|
30. |
Title: “Extraction, isolation, phytochemical screening and preliminary anti-proliferative activity of phytoconstituents of Citrullus colocynthis fruits.” Rajat Sharma (Reg. No. 18mphyto11). Thesis submitted in 2020 |
|
31. |
Title: “Analysis of trace metals in different medicinal plants by atomic absorption spectroscopy and inductively coupled plasma mass spectrometry.” Sobhana Thakur (Reg. No. 18mphyto12). Thesis submitted in 2020 |
|
32. |
Title: “In silico screening and synthesis of 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines as possible anti-inflammatory agents.” Shikha Thakur (Reg. No. 18mpharm10). Thesis submitted in 2020 |
|
33. |
Title: “Design and synthesis of 1,2,3,4-tetrahydrobenzothieno[2,3-d]pyrimidine derivatives as possible kinesin spindle protein inhibitors.” Nandini Thakur (Reg. No. 18mpharm04). Thesis submitted in 2020 |
|
34. |
Title: “In silico studies and synthesis of few 6-ethyl[2,3-d]pyrimidine derivatives as possible anti-proliferative agents.” Kaila Rushendra Kumar (Reg. No. 18mpharm07). Thesis submitted in 2020 |
|
35. |
Title: “In silico screening and synthesis of few tetrahydropyridothieno[2,3-d]pyrimidine derivatives as possible BRD4 inhibitors.” Km. Shivani (Reg. No. 18mpharm02). Thesis submitted in 2020 |
Ph. D. theses
|
1. |
Supervisor; Title: “Synthesis and anticancer evaluation of novel heterocyclics derived from imidazole and quinoline scaffolds”- PhD degree awarded to Ms. Monika Chauhan(Regn No: CUP/M.pharm-PhD/SBAS/CPS/2011-12/04) in 2017 |
|
2. |
Supervisor; Title: “Synthesis and Biological Evaluation of Inhibitors of Topoisomerases and Histone Deacetylase for In Vitro Anticancer Activity”- Ph.D.thesis submitted by Mr. Gaurav Joshi(Regn No: 15phdphm01) in May, 2019 |
|
3. |
As Co-supervisor:Title: “Oxidative Stress-Induced Cell Proliferation and DNA Repair Mechanism in glioblastoma Cells: Role of ENPP2 and APE1”- PhD degree awarded to Mr. Ravi Prakash Cholia (Regn No: CUP/MPh-PhD/SBAS/BSS/2010-11/04) in 2017 |
|
4. |
As Co-supervisor:Title: “Pharmacogenetics of Cyclophosphamide and Doxorubicin in Breast Cancer&Synthesis and Biological Evaluation of New Imidazole Based Putative Anticancer Agents”- PhD degree awarded to Mr. Sourav Kalra(Regn No: 15phdhgs03) in 2019. |
- Resource person (delivered invited lecture) in A Virtual Conference on "Confluence of Biomedical and Allied Sciences for Development of Pharmaceuticals" November 25-27, 2020; Shoolini University, Solan, HP, INDIA on the topic “EGFR Inhibitors in Anticancer Research” from November 25-27, 2020.
- Resource person (delivered invited lecture) in Workshop on spectroscopic techniques and their Biological Applications at Mata Gujri College Fatehgarh Sahib, Punjab on the topic “Mass Spectrometry” from November 2-6, 2020.
- Resource person (delivered invited lecture) at six days STTP online sponsored by AICTE on “Target Based Drug Design Strategies Utilising CADD Tools and Eco friendly Microwave Assisted Green Synthesis” ISDF college of Pharmacy, Moga, Punjab on the topic “Microwave–Assisted Green Protocols: Role in Extraction and Synthesis of Pharmaceuticals” from 27th April to 2nd May, 2020.
- Resource person (delivered invited lecture) at 5 Days Faculty Development Programme sponsored by AICTE and IKGPTU at ISDF college of Pharmacy, Moga, Punjab on the topic “Greener Processes for the construction of compounds of pharmaceutical interests” dated 16 December, 2019
- Resource person (delivered two lectures) at UGC-Human Resource Development Centre, Guru Jambeshwar University of Science and Technology, Hisar, Haryana on the topic “Liquid Chromatography & Mass Spectrometry I & II” dated 15November, 2019
- Resource person (delivered invited talk) at University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh on the topic “Modern Bioanalytical Techniques for Drug Discovery” dated 25 February – March 2, 2019
- Oral Presentation on 24th ISCB International Conference (ISCB-2018), Frontier Research in Chemistry and Biology, January 11-13, 2018, Jaipur, India
- Resource person and delivered lectures on Publication Process at ICSSR sponsored capacity Building Programme for Social Science Faculty Members, Central University of Punjab, Bathinda, May 18-31, 2018.
- Poster evaluator, 69th Indian Pharmaceutical Congress held on 22-24 December 2017 at Chitkrara University
- Oral Presentation on 7th International Conference on Stem Cells and Cancer (ICSCC-2016): Proliferation, Differentiation and Apoptosis, 21-23 October 2016, Goa India.
- Invited talk on International Conference on Drug Design, Schrodinger, April 7-9, 2017, JNU, India
- Invited Talk on Baddi University, Emerging trends in Computer aided drug design and drug delivery, 11 May 2017, Himachal, India
- Oral Presentation on 18th CRSI-National Symposium in Chemistry, CRSI-2016, 5-7 February, 2016, Chandigarh, India
- One-day program on “Acquaintance Program of Inter University Accelerator Centre, New Delhi” organised by Central University of Punjab on April 4, 2016.
- One-day program on “Prime Minister’s Fellowship Scheme for Doctoral Research” organised by Central University of Punjab on May 3, 2017.
- Invited lecture at Gurukul Kangri Vishwavidyalaya, Haridwar, India on 19-8-2016.
- One-day seminar on Evolving Importance of Intellectual Property Rights, Organised by Intellectual Property Right Cell, Central University of Punjab on January 30, 2016
- Orientation programme at Panjab University Chandigarh, Feb 10-March 09, 2015
- Refresher course at Punjabi University Patiala, May 04- May 23, 2015
- Kumar, R, M Chahuan, G Joshi and Sandeep Kumar- presented a poster at 18 CRSI, National symposium on Chemistry, 2016, held at Panjab University, Chandigarh.
- Joshi, G, Singh, S, and Kumar, R,* presented a poster entitled “Design, Synthesis and in vitro Screening of Novel Heterocycles as Potential Anticancer Agents”, International Conference onNascent Development in Chemical Sciences (NDCS-2015), organised by BITS Pilani, Rajasthan from 16th – 18th October, 2015.
- Joshi, G, Singh, S, and Kumar, R,* presented a poster at 1st International ElectronicConference on Medicinal Chemistry entitled “Design, Synthesis and in vitro Screening of Pyrazolines based compounds as Phytohaemagglutinin (PHA) mimetic” 2-27 November 2015, organized by Pharmaceuticals.
- Chauhan, M., Alex, J.M., Singh, S., Kumar, R,*Quinoline Based Inhibitors of Epidermal Growth Factor Receptor: Synthesis and In vitro Biological Evaluation, Presented on 10thInternational Symposium on Bio-Organic Chemistry, in association with International Union of Pure and Applied Chemistry (IUPAC) held at Indian Institute of Sciences Education and Research (IISER), Pune on 11-15 January, 2015.
- Invited Lecture: Kumar, R,*Pyrazolo[3,4-d]pyrimidines: Synthetic strategies and biologicalactivities, International Symposium on Recent Advances on Medicinal Chemistry (ISRAM), IL-12, NIPER, Mohali on September 8 – 10, 2014.
- Purohit, P., Seth, K., Kumar, R, Garg, S.K., Chakraborti, A.K.NOVEL HETEROCYCLIC SCAFFOLD AS COX-2 SELECTIVE, Presented on International Symposium on Recent Advances on Medicinal Chemistry (ISRAM), NIPER, Mohali on September 8 – 10, 2014.
- Invited Lecture; Kumar, R. Delivered lecture entitled “Design and Synthesis of Novel Ring Expanded Heterocycles and their Corresponding Nucleosides as Potential ChemotherapeuticAgents for Cancer and Viral Diseases” at Indian Institute of Science Education and Research(IISER),2009, Jan 23. Mohali, India
- Compounds inducing hpbmc proliferative capacity in vitro Alex, J. M., Singh, S., Kumar, R,presented at one day symposium on Recent Trend in Molecular Medicine held at CUPB on December 5, 2014 (1ST Prize).
- Design Synthesis and in-vitro screening of novel heterocycles as potential anticancer agents, Joshi.G., Singh, S., Kumar, R, presented at one-day symposium on Recent Trend in Molecular Medicine held at CUPB on December 5, 2014 (2nd Prize).
- Kaur, G, Kumar, R,* Rationale design of APE1 DNA repair inhibitors through in silico approaches, Presented on 8th Chandigarh Science Congress, CHASCON-2014 held at Panjab University, Chandigarh on 26th-28th February 2014.
- Kumar, R,* Rana, A., Chauhan, M., Singh, S., Microwave assisted synthesis of derivatives of 4-aminoquinolines as potential anticancer agents, Presented in 50th Annual Convention of Chemist 2013 held at Punjab University, Chandigarh on December 04-07, 2013.
- Chauhan, M., Alex, J. M., Singh, S., Kumar, R,* An easy and greener approach for the synthesis of novel heterocyclics and their anticancer evaluation, Presented in 50th Annual Convention of Chemist 2013 held at Punjab University, Chandigarh on December 04-07, 2013.
- Chairperson at UGC sponsored seminar on “Green Chemistry” organized at GHG Khalsa
- College, Gursar Sadhar, Punjab, February 24-25, 2012
- National Seminar on Environment and Health, September 27, 2011 held at Central University of Punjab, Bathinda.
- 3rd NIPER-(RBL)-CDRI symposium on Medicinal Chemistry and Pharmaceutical Sciences, March 03-05, 2011 held at CDRI Lucknow.
- A short term certificate course (from August 23, 2010 to August, 27, 2010) on Faculty Induction Training Programme conducted by Education and Educational Management, at National Institute of Technical Teachers’ Training and Research, Chandigarh.
- Nepali, K., Agarwal A., Kumar, R.*, Banerjee, U. C., Dhar, K. L., Suri, O. P. Design, synthesis and biological evaluation of N-(3-oxo-1,3-diaryl/dihetroarylpropyl)acetamides as potential non-purine xanthine oxidase inhibitors, Abstract published in Med. Chem. Res. 2010, 19, S77. Current Trends in Drug Discovery Research 2010, February 17-21 at Central Drug Research Institute (CDRI),Lucknow. Poster No. 55.
- Sapra, S., Sandhu, H. S., Chugh, M., Kumar, R.*,Padh, H., Shishoo, C. J., Dhar, K. L. Synthesis and antimalarial activity of arylidene derivatives of Meldrum’s acid, Abstract published in Med. Chem. Res. 2010, 19, S113. Current Trends in Drug Discovery Research 2010, February 17-21 at Central Drug Research Institute (CDRI),Lucknow. Poster No. 117.
- Kumar, S., Gupta, M., Agarwal A., Kumar, R.*, Banerjee, U. C., Dhar, K. L. Design, syntheisis and xanthine oxidase inhibitory activities of novel 5:6-fused heterocycles, Abstract published in Med. Chem. Res. 2010, 19, S126, Current Trends in Drug Discovery Research 2010, February 17-21 at Central Drug Research Institute (CDRI),Lucknow. Poster No. 139.
- Kalra, S., Sandhu, H. S., Sapra, S. and Kumar, R.*An account on biological potential of synthetics derived from Meldrum’s acid, Challenges and Opportunities for Pharmacy Graduates in21stCentuary, Indian Pharmacy Graduates’ Association (IPGA), 2009, November 7-8 at I.S.F.College of Pharmacy, Moga, Punjab, India. Poster No. B24.
- Singh, P., Gupta, M., Yadav, S. K. and Kumar, R.*Synthetic purines and pyrimidines: recent entries as potential anticancer and antiviral agents, Challenges and Opportunities for PharmacyGraduates in 21stCentuary, Indian Pharmacy Graduates’ Association (IPGA),2009, November 7-8at I.S.F. College of Pharmacy, Moga, Punjab, India. Poster No. B25.
- Kumar, R. Ujjinamatada, R. K. and Hosmane, R. S. The First Synthesis of a Novel 5:7:5-Fused Diimidazodiazepine Ring System and Some of its Chemical Properties, Zing Med. ChemConference, 2009, Feb 2-4. Playa del Carmen, Mexico.
- Khathik, G. L. Kumar, R. and Chakraborti, A. K. Co-operative Dual Activation Role of Water in Catalyst-free C-S Bond Formation, National Symposium on New Challenges in Chemistry, 2006, Mar 20 – 21. Guru Nanak Dev University, Amritsar, India Poster No. PP 23.
- Motiwala, H. F., Kumar, R. and Chakraborti, A. K. Microwave-Assisted Catalyst and Solvent-Free Synthesis of 4-Aminoaryl Derivatives of 4,7-Dichloroquinolines, National Symposium on NewChallenges in Chemistry, 2006, Mar 20–21. Guru Nanak Dev University, Amritsar, India PosterNo. PP 24.
- KamleshMeena, Kumar, R. Pankaj Soni, Asit K. Chakaraborti and U.C. Banerjee. Chemoenzymatic synthesis of key precursor of (S)-sotalol, Department of Biotechnology, Punjabi University, Patiala, 21 -22, March, 2006.
- Kumar, R. and Chakraborti, A. K. An Efficient Protocol for acetal formation under the catalyticinfluence of Copper (II) Tetrafluoroborate hydrate, National Symposium on Chemistry, 8th CRSI 2006, Mumbai (India).
- Kumar, R., Thilagavathi, R., Aparna V., Sobhia, M. E., Gopalakrishnan, B. and Chakraborti,A. K. 3-D QSAR Studies on Imidazolyl and N-PyrrolylHeptenoates as HMG-CoA Reductase Inhibitors, National Symposium on Chemistry, 7th CRSI 2005, Kolkata (India).
- K. S., Kumar, R. and Chakraborti, A. K. Novel Transition Metal Derived Catalyst for Thia-MichealReaction.National Symposium on Chemistry, 7th CRSI 2005, Kolkata (India).
- Chakraborti, A. K., Kondaskar, A., Kumar, R. and Rudrawar, S. Complementarity of Zeolites and Clays in Catalyzing Nucleophilic Opening of Epoxides: Applications for Synthesis of Drug and Drug Intermediates, ICOB-4 & ISCNP-24 (IUPAC International Conference on Biodiversity and Natural Products: Chemistry and Medical Applications), January 2004, Delhi (India) P-165.
- Chakraborti, A. K., Kaur, G., Sharma, L., Magesh, S. and Kumar, R. Solution and Solid Phase Combinatorial Synthesis of Stilbene Libraries, Biotechnology-A Challenge to PharmacyProfession, 54thIndian Pharmaceutical Congress, December 2002, Pune (India) B1-27.
- Organised three day workshop on “Drug Design, Molecular Docking, Virtual Screening and Pharmacoinformatics” in association with Schrödinger INC. USA from 26-11-2015 to 28-11-2015.
- Organised a three-day workshop on “Advanced workshop on molecular docking, virtual screening and computational biology” in association with Schrödinger INC. USA from 15-11-2017 to 17-11-2017.
1. Lead Guest Editor of Special Issue on “Signal Transduction Inhibitors as Promising Anticancer Agents” BioMed Research International(http://www.hindawi.com/journals/bmri/si/636318/cfp/)
2. Associate Editor: Frontiers in Behavioral Neuroscience; Mini-Reviews in Organic Chemistry
3. Reviewer to the following Journals and Grant agencies:
Angewandte Chemie (Wiley), Medicinal Research Reviews (Wiley), Future Medicinal Chemistry, ACS Chemical Neuroscience, Journal of Organic Chemistry (JOC, ACS), Journal of Medicinal Chemistry (J Med Chem, ACS), Journal of Agricultural and Food Chemistry (ACS), RSC Advances (RSC), Molecular Biosystems (RSC, MedChemCom (RSC), Bioorganic Chemistry (Elsevier), Bioorganic and Medicinal Chemistry (BMC, Elsevier), Bioorganic and Medicinal Chemistry Letters (BMC, Elsevier),Food and Chemical Toxicology (FCT, Elsevier), European Journal of Medicinal Chemistry (EJMC, Elsevier), International Journal of Biological Macromolecules (Elsevier), Medicinal Chemistry Research (Springer), Mini Reviews in Medicinal Chemistry (Benthem), Journal of Enzyme Inhibition and Medicinal Chemistry (Informa healthcare), Letters in Drug Design & Discovery (Bentham science), Biomarkers in Cancer (Libertas Academica), Drug Target Insights (Libertas Academica), PLosOne, Chemistry and Biology, Wiley, Organic Chemistry Insights (Libertas Academica), Grant agency: Czech Republic Foundation, Grant agency: Department of Science and Technology, DST, SERB, CSIR and AICTE
PATENT FILED
1. Title: Indazolo[2,3-c]quinazoline based fluorophores and their applications in Bio-imaging and Tagging: Kumar, R., Singh, S,. Joshi, G,. Sharma P. Indian Patent application no. 201811028230 filed on July 27, 2018.
2. Title: Novel fused heterocycles and method of use and manufacture thereof. Inventors: Kumar, R., Singh, S,. Chauhan, M. Indian Patent application no. 201611014161 filed on April 22, 2016.
3. Title: Fused diimidazodiazepine compounds and methods of use and manufacture thereof. Inventors: Hosmane, R. S., Raman, V and Kumar, R.U.S. Patent No. 8,518,901. Washington, DC: U.S. Patent and Trademark Office.
4. Title: Novel Cyclooxygenase-2 Inhibitors. Inventors: Chakraborti, A. K. Banerjee, U. C., Kumar, R., Garg, S. K., Meena, V. S., Indian Patent Grant No. 283941; Grant Date: 06-06-2017. Indian patent, application no. 638/DEL/2008 filed on 14th March 2008.
5. Title: Inhibitors of Phosphodiesterase Type-IV: Benzimidazolone series. Inventors: Chakraborti, A. K. Sarin, S., Rudrawar, S., Kumar, R., Chankeshwara, S. V., Dastidar, S., Ray, A. European patent, application no. 08151539.7-2117, filed on 15th February 2008. US patent, application no. 12/031842, filed on 15th February 2008, United States Patent 20080207659
6. Title: Phosphodiesterase-4 Inhibitors: Urea, Carbamate and Amide series. Inventors: Chakraborti, A. K. Sarin, S., Rudrawar, S., Kumar, R., Chankeshwara, S. V., Dastidar, S., Ray, A. Indian patent, application no. 565/DEL/2006, filed on 3rd March 2006.
7. Title: Quinoline bridged hydroxamate-based anticancer agents and the method for preparation thereof: Kumar, R., Singh, S,. Joshi, G. Indian Patent application no. 202211053705 dated September 20, 2022.
PATENT PUBLISHED
1. Title: Fused diimidazodiazepine compounds and methods of use and manufacture thereof. Inventors: Hosmane, R. S., Raman, V and Kumar, R.U.S. Patent No. 8,518,901. Washington, DC: U.S. Patent and Trademark Office.
2. Title: Novel Cyclooxygenase-2 Inhibitors. Inventors: Chakraborti, A. K. Banerjee, U. C., Kumar, R., Garg, S. K., Meena, V. S., Indian Patent Grant No. 283941; Grant Date: 06-06-2017. Indian patent, application no. 638/DEL/2008 filed on 14th March 2008.
3. Title: Inhibitors of Phosphodiesterase Type-IV: Benzimidazolone series. Inventors: Chakraborti, A. K. Sarin, S., Rudrawar, S., Kumar, R., Chankeshwara, S. V., Dastidar, S., Ray, A. European patent, application no. 08151539.7-2117, filed on 15th February 2008. US patent, application no. 12/031842, filed on 15th February 2008, United States Patent 20080207659
4. Title: Phosphodiesterase-4 Inhibitors: Urea, Carbamate and Amide series. Inventors: Chakraborti, A. K. Sarin, S., Rudrawar, S., Kumar, R., Chankeshwara, S. V., Dastidar, S., Ray, A. Indian patent, application no. 565/DEL/2006, filed on 3rd March 2006.
Total citations: 3230
h Index = 36 (Google scholar)
i10 index: 62

![Graphical abstract: Cyclocondensation reactions of an electron deactivated 2-aminophenyl tethered imidazole with mono/1,2-biselectrophiles: synthesis and DFT studies on the rationalisation of imidazo[1,2-a]quinoxaline versus benzo[f]imidazo[1,5-a][1,3,5]triazepine selectivity switches](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00706C&imageInfo.ImageIdentifier.Year=2018)